Uncountable’s Chemical Search tool allows users to efficiently search for specific molecules within the platform’s database by scanning ingredients or experiments that include chemical structures. This feature streamlines the process of finding and analyzing compounds. To access the tool, press Control + K and type “Chemical Search.”
From the Chemical Search page, users can search for molecules within the platform based on a SMILES string or chemical structure drawing. Search results can be filtered or limited by specifying search type and similarity levels.

Filters
First, add an optional filters to limit your search (1). Like listing filters, Chemical Search filters can apply to all entities within the platform. For example, by adding a Experiment Creator filter, users could search through only experiments created by a specific individual.
Search Type
Then, refine your search by selecting one of three search types (2):
- Equality: Results include only exact matches of the chemical structure or SMILES string.
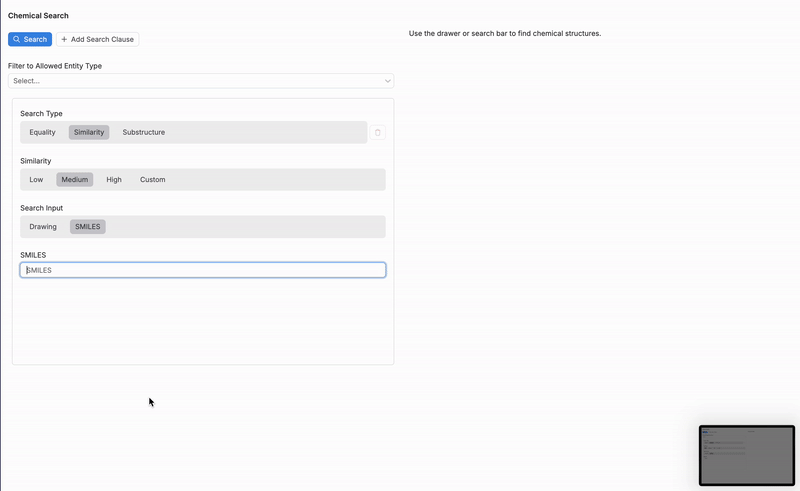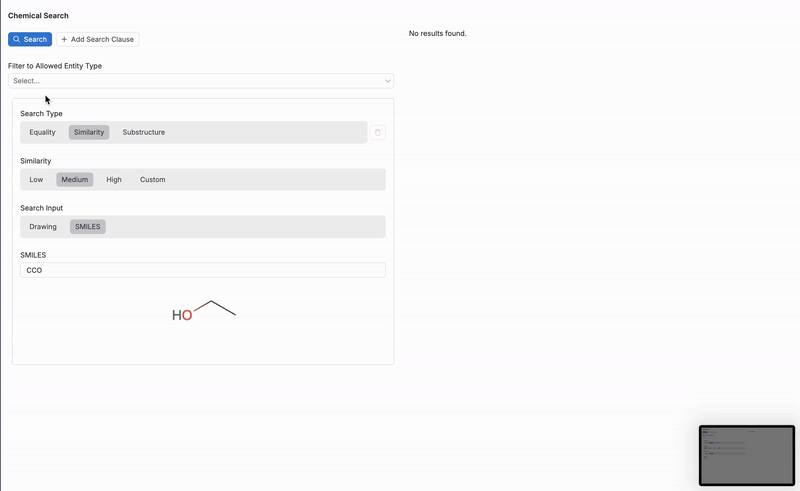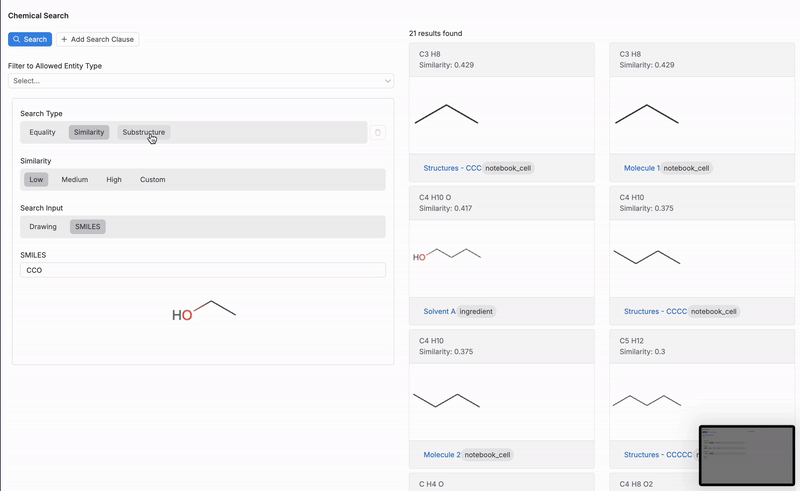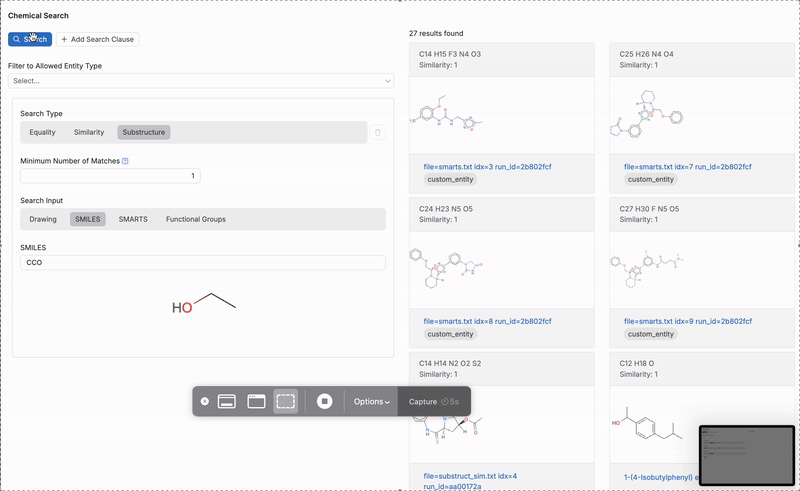
- Similarity: Results include molecules of Low, Medium, High, or Custom similarity.
- Substructure: Results include molecules containing the chemical structure or SMILES string. When using a this search type, users can also input SMARTS or functional group search inputs.
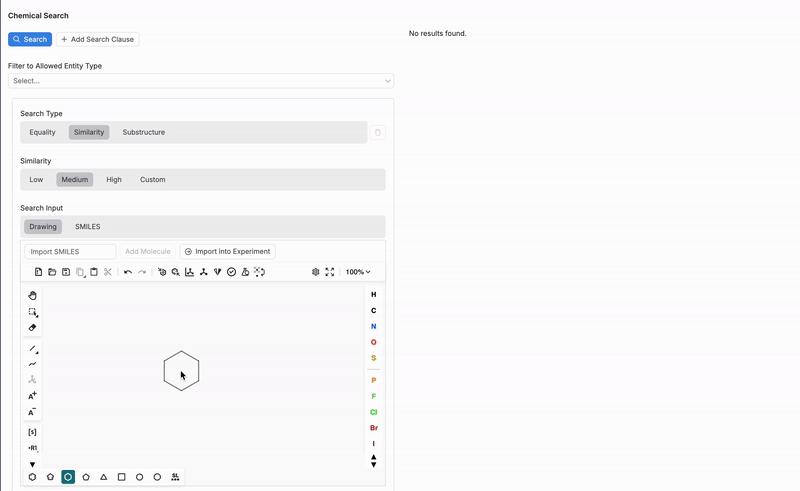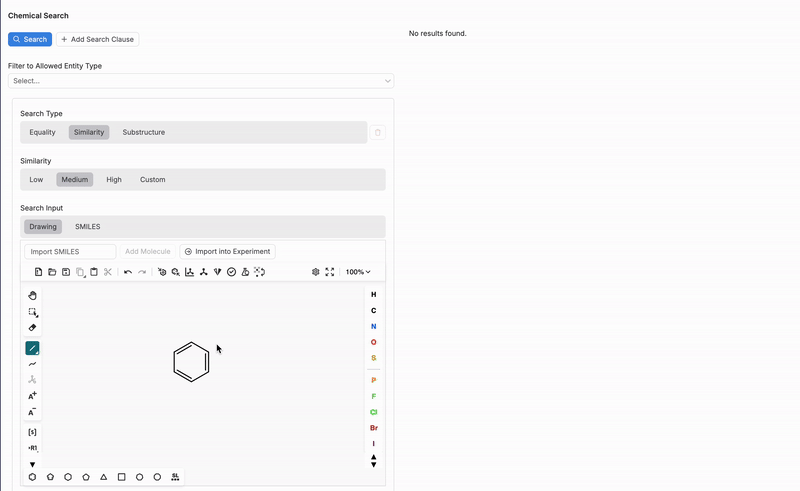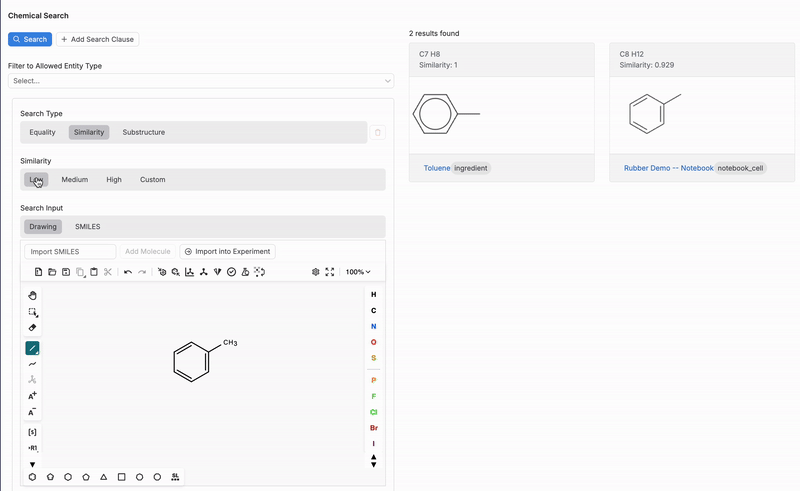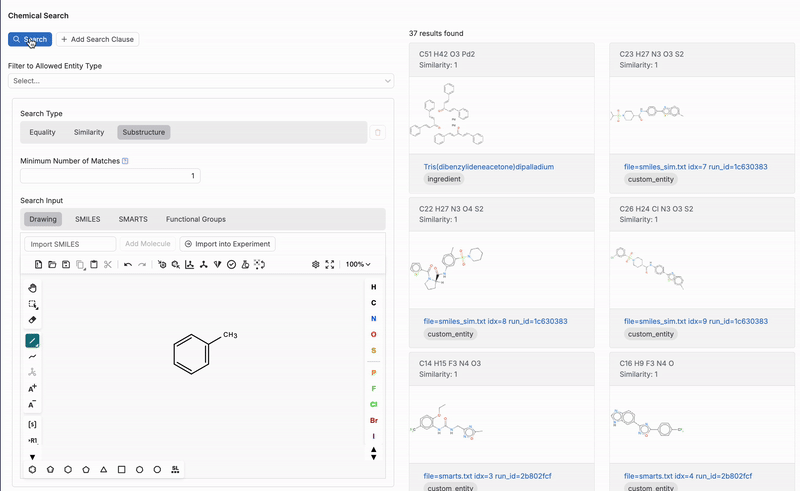
Similarity
When using the Similarity search, users can specify the number of results to return, based on their similarity to the input chemical structure or SMILES string (3).
- Low: Results include molecules with a low similarity and above.
- Medium: Results include molecules with a medium similarity and above.
- High: Result include only molecules with a high similarity.
- Custom: Input a custom similarity level (i.e. inputing 0.8 will result in only molecules with an 80% similarity and above).
Search Input
Next, select a search input type (4):
- Drawing: Use the Drawing tool to search for molecules based on their similarity to a chemical structure. You can also use it to locate a molecule by drawing a substructure.
- SMILES: Use the SMILES input to search for molecules based on their exact structure. You can also use string inputs to find molecules that contain a specific substructure.
Drawing
If using the Drawing search input, users can utilize the interactive drawing tool at the bottom of the Chemical Search page to sketch out a chemical structure or substructure. The drawing tool supports a variety of chemical symbols and bonds, allowing users to construct molecules in detail.
Once a structure is drawn, the tool will search for matches or similar molecules within the platform’s database based on the search type and similarity level selected. This feature is especially useful for identifying molecules with specific functional groups or similar structural patterns.
SMILES
If using the SMILES search input, users can input the exact SMILES (Simplified Molecular Input Line Entry System) string of a molecule to search for a specific structure. SMILES strings offer a linear representation of molecular structures, which can be used to find exact matches, substructures, or molecules with similar compositions. This method is ideal for users who already have a known SMILES string or want to identify molecules by their precise arrangement.
An example of a SMILES string for ethanol (a simple alcohol) is:
CCO
- C represents a carbon atom.
- O represents an oxygen atom.
- The two C‘s represent the two carbon atoms in the ethanol molecule, and O represents the hydroxyl group (-OH) attached to the second carbon.